Healthy Ageing
Targeting SARS-CoV-2 PLpro protease blocks virus spread
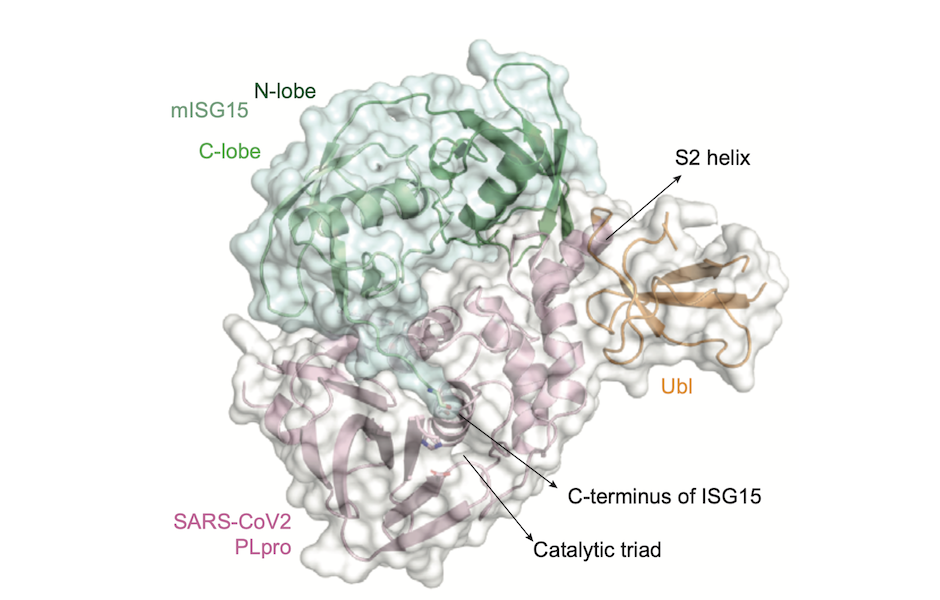
From proposal to Nature paper in one month, rapid access experiments supporting Covid-19 research demonstrate a potential route to block coronavirus spread.
Deciphering the structure of proteins down to the level of individual atoms is a PSI speciality. If the exact structure of viral components is known, researchers can more easily develop drugs to help defeat the virus.
When the SARS-CoV-2 virus penetrates human cells, it lets the human host cell produce proteins for it. One of these viral proteins, called PLpro (papain-like protease), is essential for the virus to multiply and spread.
One of the reasons the SARS-CoV-2 virus is so successful – and thus dangerous – is that it can suppress the non-specific immune response.
In the case of an infection, the SARS-CoV-2 virus must overcome various defence mechanisms of the human body, including its non-specific or innate immune defence. During this process, infected body cells release messenger substances known as type 1 interferons. These attract natural killer cells, which kill the infected cells.
PLpro has two functions: it plays a role in the maturing and release of new virus molecules, and it suppresses the development of type 1 interferons.
This research has demonstrated that pharmaceutical targeting of PLpro by a non-covalent inhibitor (GRL-0617) blocks virus spread and increases anti-viral immunity in human epithelial cells, the prime site of pathogen entry.
The further development of PLpro-inhibiting substance classes for use in clinical trials is now a key challenge for this therapeutic approach.
Structure solution at the Swiss Light Source
The structural biology work to determine the interlocking of the GRL-0617 inhibitor with PLPro was performed at the macromolecular crystallography beamline X06SA-PXI. The Nature paper was submitted within one month of submitting a research proposal to the "Priority Covid-19 Call” for rapid access to synchrotron analysis: crystallographic data collection happened on the 9th of April 2020, and results were published on the 29th of July 2020.
The Frankfurt-based research team made full use of the remote access facilities at the Swiss Light Source, avoiding the need to travel during the pandemic. The crystals were sent by specialist courier service and loaded onto the measuring station by a PSI scientist, who then enabled the beamline for full remote control of the experiments.
References
Shin D. et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity.
Nature. 2020;s41586-020-2601-5.
https://doi.org/10.1038/s41586-020-2601-5
