Featured Projects
Mapping sunlight induced chemical reactions in aerosol particles

The Swiss Light Source is a unique location for discovering groundbreaking physics and chemistry happening in the atmosphere.
Atmospheric aerosol particles are known to have a large impact on climate, air quality, and human health.
Ranging in size from nanometres up to tens or hundreds of micrometres, they can survive from days to weeks, and can travel over long distances.
As aerosol particles have such a wide impact on our environment and health, it is essential to investigate their size, composition, distribution, and relevant physical and chemical processes.
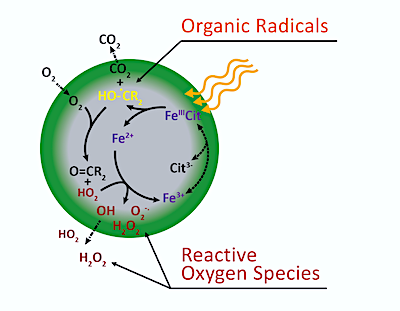
In organic rich aerosol particles containing iron, sunlight can induce low oxygen conditions that stabilize reactive oxygen species (ROS) and carbon-centered radicals (CCRs) that can take part in chemical reactions.
Mapping aerosol chemistry in real time
The Pollux instrument at the Swiss Light Source offers a unique facility worldwide for x-ray synchrotron analysis of aerosol particles. A photochemical environmental cell can be used to study light induced chemistry over many hours in controlled atmospheric conditions.
With 35 × 35 nm2 spatial resolution, in situ photochemical studies using x-ray spectromicroscopy can detect fine differences in the electronic structure of molecules in the particles, oxidation state of trace metals, or their specific carbon, oxygen and nitrogen functionalities. This compositional and spatial information gathered in real time yields in situ chemical mapping of particles with a time resolution of a few minutes.
Aerosol chemistry altered by sunlight
Wind blown mineral dust is the main source of atmospheric iron. Organic acids can promote its dissociation into soluble iron, which then participates in chemical processes in the aerosol particles.
This pioneering study used particles containing a mixture of iron(III)-citrate and citric acid, mimicking iron organic complexes in ambient aerosol particles.
In sunlight, the iron(III)-citrate/citric acid complex breaks apart generating iron(II), carbon centered radicals and CO2.
X-ray imaging shows that oxygen reactions occur near particle surfaces, but not in their centres. This is because oxygen does not penetrate such viscous particles due to the combined effect of fast reaction and slow diffusion near the particle surface, causing photochemically produced radicals to be effectively trapped in the aerosol particle.
This research shows why scientists have not yet had a full picture of aerosol chemistry - they have never considered particle viscosity and sunlight driven reactions at the same time. It shows that ROS and CCRs can exist in aerosols at higher concentrations than previously known and for longer times. It also suggests why health effects can often be more severe than expected when aerosol particles are inhaled.
REFERENCES
Photolytic radical persistence due to anoxia in viscous aerosol particles
Alpert PA, Corral Arroyo P, Schneider F, et al.
Nature Communications 12, 1769 (2021)
https://doi.org/10.1038/s41467-021-21913-x
