Featured Projects
How catalysts age

Local chemistry and structural changes quantified from the micrometer to the atomic level.
Clariant AG have used a novel combination of spectroscopy and tomography to non-destructively image the interior of pristine and used catalyst pellets with nanometre resolution.
The used vanadium phosphorus oxide (VPO) pellets were sourced from an industrial fixed-bed reactor after 4 years of known operation history.
During reactor operation, the catalyst undergoes a series of structural and compositional changes that culminate in a gradual loss of catalyst productivity.
Working with tomography experts at the Swiss Light source, high-resolution 3-D reconstructions of new and used pellets showed the transformation from a mesoporous catalyst of high surface area, composed of a series of amorphous and nanocrystalline vanadium phosphate phases, to a macroporous catalyst composed of micrometre sized and defect rich vanadyl pyrophosphate crystals.
Local chemistry within 26 nanometre voxels was mapped to give electron density, vanadium concentration, and the degree of oxidation of the vanadium.
It was already known that the relative content of vanadium decreased over time. But synchrotron analysis was uniquely able to pinpoint where in the crystal lattice these atoms were missing, and confirm that these holes in the atomic lattice can serve as additional active sites for the process of catalysis.
These observations demonstrate the power of synchrotron analysis to influence the design of catalysts to make them more durable.
Sparse XTNES - an efficient measuring strategy
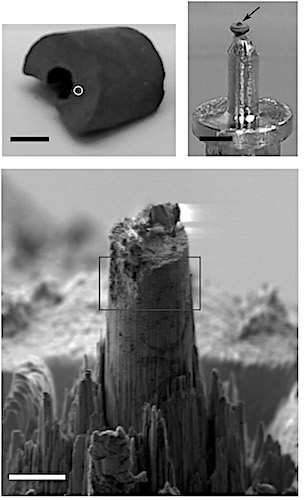 Measurements used the cSAXS instrument at the Swiss Light Source and combined spectroscopy and tomography in a technique known as sparse x-ray transmission near-edge spectrotomography - XTNES. The sparse sampling approach reduced experimental time per XTNES tomogram from more than 1 week to less than 20 hours.
Measurements used the cSAXS instrument at the Swiss Light Source and combined spectroscopy and tomography in a technique known as sparse x-ray transmission near-edge spectrotomography - XTNES. The sparse sampling approach reduced experimental time per XTNES tomogram from more than 1 week to less than 20 hours.
About VPO catalysts
Vanadium phosphorus oxides (VPOs) are widely used as catalysts. One use is in the selective oxidation of n-butane to maleic anhydride (C4H2O3) and its chemical derivatives maleic acid and fumaric acid. These chemical intermediates find use in nearly all areas of chemistry, most prominently in the production of unsaturated polyester resins for household and industrial plastics. Over 3 million metric tonnes of maleic anhydride are produced annually.
Industrial conversion is frequently carried out in fixed-bed reactors, operating at roughly 400°C. These reactors consist of a series of actively cooled reactor tubes (typically more that 10,000 tubes with an inner diameter of ~20 mm) filled with porous VPO catalyst bodies or pellets. Pressurized, preheated mixtures of air, n-butane (< 2 vol.%), and water (< 3 vol.%) are continuously fed through the reactor tubes from the bottom. Reaction products are siphoned off from the top.
References
Sparse ab initio X-ray transmission spectro-tomography for nanoscopic compositional analysis of functional materials
Z. Gao, M. Odstrcil, S. Böcklein, D. Palagin, M. Holler, D. Ferreira Sanchez, F. Krumeich, A. Menzel, M. Stampanoni, G. Mestl, J.A. van Bokhoven, M. Guizar-Sicairos, J. Ihli
Science Advances 2021; 7 : eabf6971
https://www.science.org/doi/10.1126/sciadv.abf6971
