Chemistry
Tracking nanoparticle reactions in a catalyst
A powerful combination of research techniques opens up a new dimension for nanoscale investigation of catalytic processes.
A demonstration experiment completed at the Surfaces/Interfaces Microscopy (SIM) beamline at the Swiss Light Source opens up new avenues for the investigation and understanding of catalytic processes.
The results from studying fundamental processes in supported catalysts suggest that industrial production processes can be optimised in a targeted way.
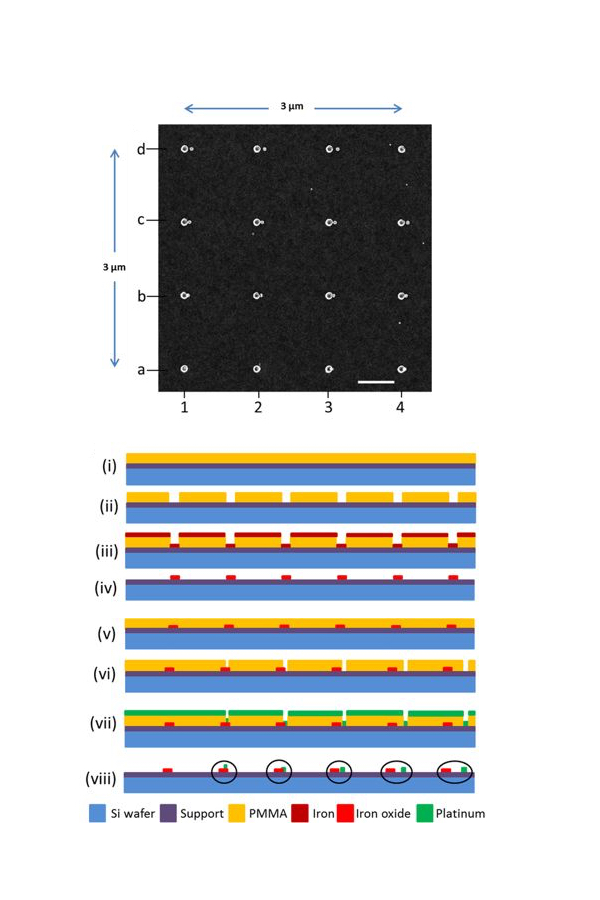
The new method rests on three pillars:
- nano-fabrication of a model system using electron beam lithography
- the precise simultaneous measurement of the chemical reactions
- data modelling using advanced calculation techniques
The demonstration experiment successfully evaluated the hydrogen spillover reduction of iron oxide using a platinum catalyst on different substrates.
Using electron beam lithography multiple pairs of iron oxide and platinum nanoparticles were placed on titanium oxide and aluminium oxide supports with a precision of one nanometre. The distance between the pairs varied from zero to 45 nanometres.
Using single-particle in situ x-ray absorption spectromicroscopy applied simultaneously to all particle pairs, the extent of the reduction of the iron oxide particles by hydrogen atoms generated on the platinum was recorded.
In the reaction, hydrogen gas flowing over the surface is split by platinum nanoparticles into elemental hydrogen that flows down the sides and across the support material to react with iron oxide.
For the aluminium oxide support, which itself cannot be reduced, the hydrogen flows no further than 15 nanometres from the platinum nanoparticle.
In contrast, for the reducible titanium oxide support, hydrogen flows over the whole surface and the reaction is ten orders of magnitude more efficient than on aluminium oxide.
The ability to construct catalytic model systems accurate to one nanometre and then to track the chemical reactions of individual nanoparticles makes it possible for the chemical industry to selectively investigate and optimise the efficiency of catalytic processes.
References
Catalyst support effects on hydrogen spillover
Waiz K, Clelia S, Armin K, Jens G, Van de Vondele J, Ekinci Y, van Bokhoven JA
Nature. 2017; 541:68-71.
https://doi.org/10.1038/nature20782
